RECENT product recalls
Joly’s vinegar recall
Necessary Information:
See original announcement for examples of labels
Company Announcement:
Name of Product:
Joly’s 80% Vinegar (32 oz, pack of two)
Hazard:
The recalled vinegar product violates the precautionary labeling requirements under the Federal Hazardous Substances Act (FHSA) because the hazardous substance lacks required warning labels and first-aid treatment instructions. This poses a deadly risk of poisoning if the contents are swallowed and a serious injury from chemical burns to the skin and eyes if improperly handled.
Remedy:
Refund
Recall Date:
February 19, 2026
Units:
About 450
Consumer Contact: Joly’s at 240-412-8720 from 9 a.m. to 5 p.m. ET Monday through Friday or email at jolysllc@gmail.com.
Description:
This recall involves Joly’s 80% Vinegar (32-ounce, pack of two). The vinegar comes in a white capped transparent bottle with an orange and red label with white lettering that has “Joly’s” brand, “80% Vinegar” and “Dilutes to 5 Gallons”. There are no other markings on the container.
Remedy:
Consumers should move the vinegar products out of sight and reach of children immediately and contact Joly’s to receive a full refund and disposal instructions. Consumers will be asked to send an email to jolysllc@gmail.com with a photo of the recalled product in its original packaging and “RECALLED” printed on the container.
Incidents/Injuries:
None reported
Sold Online At:
Amazon.com from May 2025 through November 2025 for about $23.
Manufacturer(s):
Joly’s LLC, of Orlando, Florida
Retailer:
Joly’s LLC, dba Josie’Store, of Orlando, Florida
Manufactured In:
United States
Recall number:
26-273
Alkaloids Chewable Tablets recall
Necessary Information:
See original announcement for examples of labels
Company Announcement
FOR IMMEDIATE RELEASE – February 13, 2026 – North Kansas City, Missouri, Shaman Botanicals, LLC is voluntarily recalling one lot (Lot B# AAW.501.3) of Alkaloids Chewable Tablets—White Vein to the consumer level. Recent testing showed that the Alkaloids Chewable Tablets—White Vein product contains 7-Hydroxymitragynine (7-OH) in an amount more than the declared value of 7.5 mg/tablet.
Risk Statement: Use of the Alkaloids Chewable Tablets—White Vein product could result in consumers ingesting a higher dose than intended, which could result in adverse health effects.
Shaman Botanicals, LLC has not received any reports of adverse events related to this lot of Alkaloids Chewable Tablets—White Vein.
The product is packaged in a 2-count bag with UPC Code 810057763724, in a 20-count bag with UPC Code 810057763830 and in a 30-count bottle with UPC Code 810057763779. The affected Alkaloids Chewable Tablets—White Vein lot has the following lot number: Lot B# AAW.501.3 (all expiration dates). The product can be identified by the lot number on the package and/or the bottom of the bottle. The Alkaloids Chewable Tablets—White Vein product was distributed nationwide to wholesalers, retailers, and consumers via online sales.
Shaman Botanicals, LLC is notifying its distributors and customers by email and is arranging for return of the affected product and providing refunds or replacement of all recalled products. Wholesalers, distributors, retailers, and consumers that have the Alkaloids Chewable Tablets—White Vein product that is being recalled should quarantine the product, stop using it, and return it to Shaman Botanicals, LLC.
To receive a full refund or replacement product, please go to the attached URL to register your return. https://recall.cbdamericanshaman.com/External Link Disclaimer.
Consumers with questions regarding this recall can contact Shaman Botanicals, LLC, Monday through Friday from 9am-5pm CST, at:
Vince Sanders c/o Quality Department
1501 Iron Street
North Kansas City, MO 64116
Telephone: 855-427-7386
Email: Quality@CBDAmericanShaman.com
https://recall.cbdamericanshaman.com/External Link Disclaimer
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this dietary supplement product.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail, or by fax.
Complete and submit the report online: www.fda.gov/medwatch/report.htm
Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
Bremer FAMILY SIZE ITALIAN STYLE MEATBALLS recall
Necessary Information:
See original announcement for examples of labels
Company Announcement:
WASHINGTON, FEB. 22, 2026 – Rosina Food Products, Inc., a West Seneca, N.Y. establishment, is recalling approximately 9,462 pounds of ready-to-eat (RTE) frozen meatball products that may be contaminated with foreign material, specifically metal, the U.S. Department of Agriculture’s Food Safety and Inspection Service (FSIS) announced today. The RTE fully cooked frozen meatball products were produced on July 30, 2025. The following products subject to the recall are [view labels]:
32-oz. printed poly film bag packages of fully cooked frozen “Bremer FAMILY SIZE ITALIAN STYLE MEATBALLS” containing “about 64 meatballs per package” with “BEST BY” date of “10/30/26” with timestamps between 17:08 through 18:20 printed on the back of the label.
The products subject to recall bear establishment number “EST. 4286B” inside the USDA mark of inspection. The affected products have a 15-month shelf-life and were shipped to Aldi supermarket locations nationwide.
The problem was discovered after FSIS received a consumer complaint regarding metal fragments found within the product.
There have been no confirmed reports of injury due to consumption of these products. Anyone concerned about an injury should contact a health care provider.
FSIS is concerned that some product may be in consumers’ refrigerators or freezers. Consumers who have purchased these products are urged not to consume them. These products should be thrown away or returned to the place of purchase.
FSIS routinely conducts recall effectiveness checks to verify recalling firms notify their customers of the recall and that steps are taken to make certain that the product is no longer available to consumers. When available, the retail distribution list(s) will be posted on the FSIS website at www.fsis.usda.gov/recalls.
Members of the media and consumers with questions regarding the recall can contact Customer Service, Rosina Food Products, Inc., at 1-888-767-4621 or CService@rosina.com.
Consumers with food safety questions can call the toll-free USDA Meat and Poultry Hotline at 888-MPHotline (888-674-6854) or send a question via email to MPHotline@usda.gov. For consumers that need to report a problem with a meat, poultry, or egg product, the online Electronic Consumer Complaint Monitoring System can be accessed 24 hours a day at https://foodcomplaint.fsis.usda.gov/eCCF/.
**************RECALL************
Necessary Information:
Web Link- https://www.fsis.usda.gov/.../ajinomoto-foods-north...
See original announcement for examples of labels
Company Announcement:
WASHINGTON, FEB. 19, 2026 – Ajinomoto Foods North America, Inc., a Portland, Ore. establishment, is recalling approximately 3,370,530 pounds of frozen not ready-to-eat (NRTE) chicken fried rice products that may be contaminated with foreign material, specifically glass, the U.S. Department of Agriculture’s Food Safety and Inspection Service (FSIS) announced today.
The NRTE chicken fried rice items were produced between September 8, 2025, and November 17, 2025. The following products are subject to recall [view labels]:
1.53-kg. cardboard packages containing 6 bags of frozen “AJINOMOTO YAKITORI CHICKEN WITH JAPANESE-STYLE FRIED RICE” with BEST BEFORE/MEILLEUR AVANT dates 26 SE 09 through 26 NO 12.
20-oz. (1 lb. 4 oz.) plastic bag packages containing frozen “TRADER JOE’S Chicken Fried Rice with stir fried rice, vegetables, seasoned dark chicken meat and eggs” with BEST BY dates 9/8/2026 through 11/17/2026.
The products subject to recall bear establishment number P-18356 inside the USDA mark of inspection. The Trader Joe’s item was shipped to Trader Joe’s retail locations nationwide. The Ajinomoto item was exported only to Canada.
The problem was discovered after the establishment notified FSIS that it received four consumer complaints regarding glass found in product. There have been no confirmed reports of injury due to consumption of this product. Anyone concerned about an injury should contact a healthcare provider.
FSIS is concerned that some product may be in retailers’ or consumers’ freezers. Consumers who have purchased these products are urged not to consume them. These products should be thrown away or returned to the place of purchase.
FSIS routinely conducts recall effectiveness checks to verify recalling firms notify their customers of the recall and that steps are taken to make certain that the product is no longer available to consumers. When available, the retail distribution list(s) will be posted on the FSIS website at www.fsis.usda.gov/recalls.
Members of the media with questions about the recall can contact Corporate PR at MediaInquiry@ajinomotofoods.com or call (909) 477-4800. Consumers with questions about the recall can contact Consumer Affairs, Ajinomoto Foods North America, at (855) 742-5011 or email at customercare@ajinomotofoods.com.
Consumers with food safety questions can call the toll-free USDA Meat and Poultry Hotline at 888-MPHotline (888-674-6854) or send a question via email to MPHotline@usda.gov. For consumers that need to report a problem with a meat, poultry, or egg product, the online Electronic Consumer Complaint Monitoring System can be accessed 24 hours a day at https://foodcomplaint.fsis.usda.gov/eCCF/.
“Tippy Toes” brand Apple Pear Banana Fruit puree recall
Necessary Information:
See original announcement for examples of labels
Company Announcement:
Sanger, CA – February 13, 2026 – IF Copack LLC d.b.a. Initiative Foods (“Initiative Foods”) is recalling one lot of the “Tippy Toes” brand Apple Pear Banana Fruit puree (“Product”) due to elevated levels of patulin. Patulin is a naturally occurring substance (called a mycotoxin) which is produced by molds that may grow in various fruits, including apples. Long-term exposure resulting from ingestion of patulin can lead to various adverse health consequences, including a potential for immune suppression, nerve damage, headache, fever, and nausea. No illnesses or injuries have been reported to date.
The Product was distributed nationwide in retail grocery stores in all U.S. states other than Alaska. The Product may have been distributed in the U.S. territories of Guam and Puerto Rico.
Product Information
Product Name
Packaging Format
UPC(s)
Lot Number(s) / Expiration Date
Package Code Identifier
Tippy Toes Apple Pear Banana
2-pack plastic tubs, with product information sleeve
036800
265783
Lot # 07174
Best By Date:
“BB 07/17/2026”
INIA0120
The “Best By” (expiration) date is found on the bottom of each plastic tub. The recalled Product will have a date stamped as “BB 07/17/2026.” The package is also marked with the package code “INIA0120” as shown below:
The recalled Product was sampled under the Total Diet Study by the U.S. Food and Drug Administration (“FDA”), which found elevated patulin levels higher than is common for these products. Initiative Foods worked with FDA to identify the single lot to recall due to possible health concerns identified in this notice.
This recall is being conducted based on FDA’s recommendation.
Action for Consumer
Consumers with the Product matching the “Best By” (expiration) date of July 17, 2026 should:
Discontinue use of the Product and dispose of it immediately or return to their place of purchase for a refund.
Contact their healthcare provider if concerns arise regarding health after consumption of the Product.
Action for Retailers
Retailers should check inventory and shelves, and immediately remove the affected lot from sale or distribution and catalogue the recalled product.
Company Statement
“At Initiative Foods, the safety of our consumers and their families is our highest priority. We are cooperating with the FDA to ensure strict review and enhanced safety measures across all our products. We thank our retail partners and customers for their understanding and prompt action on this matter,” said Don Ephgrave, Initiative Foods’ CEO and President.
More Information
For further recall information and updates, consumers and retailers can call a dedicated toll-free number: 1-855-215-5730, Monday through Friday, 8 a.m. to 5 p.m. Eastern Standard Time.
This recall is being conducted with the full knowledge of the U.S. Food and Drug Administration.
Rosabella Moringa Capsules recall
Necessary Information:
FDA Outbreak Investigation: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-extensively-drug-resistant-salmonella-moringa-powder-february-2026
CDC Outbreak Investigation: https://www.cdc.gov/salmonella/outbreaks/moringacapsules-02-26/index.html
See original announcement for examples of labels
Company Announcement
Ambrosia Brands, LLC of New York, New York is recalling certain lots of its Rosabella Moringa Capsules product due to possible contamination with Salmonella, an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Healthy persons infected with Salmonella often experience fever, diarrhea (which may be bloody), nausea, vomiting and abdominal pain. In rare circumstances, infection with Salmonella can result in the organism getting into the bloodstream and producing more severe illnesses such as arterial infections (i.e., infected aneurysms), endocarditis and arthritis.
The recalled product was sold to consumers located nationwide in the United States, through our direct-to-consumer website (tryrosabella.com) and Tik Tok Shop (https://www.tiktok.com/shop/pdp/moringa-capsules-800mg-by-rosabella-for-immune-gut-health-support/1729464033034539782External Link Disclaimer), since February 2025. We are also aware that there may be unauthorized 3rd party distribution to consumers through ebay.com, Shein, or other sites. None of the impacted lots were sold by us on Amazon.com, however, while we do not have any authorized resellers on Amazon.com, we urge you to check your lot numbers for any Rosabella Moringa capsules purchased on the site.
The recall includes the following product and lots:
Rosabella Moringa Capsules, 60 Count Bottles
Lots impacted (all start with 1356 as the sku number, and end in a -1 or -2 after the lot code):
Lot
Expiration Date
5020591
03/2027
5020592
03/2027
5020593
03/2027
5020594
03/2027
5020595
03/2027
5020596
03/2027
5030246
04/2027
5030247
04/2027
5030248
04/2027
5030249
04/2027
5030250
04/2027
5030251
04/2027
5040270
05/2027
Lot
Expiration Date
5040271
05/2027
5040272
05/2027
5040273
05/2027
5040274
05/2027
5040275
05/2027
5040276
05/2027
5040277
05/2027
5040278
05/2027
5040279
05/2027
5050053
6/2027
5050054
6/2027
5050055
6/2027
5050056
6/2027
Lot
Expiration Date
5060069
07/2027
5060070
07/2027
5060071
07/2027
5060072
07/2027
5060073
07/2027
5060074
07/2027
5060075
07/2027
5060076
07/2027
5060077
07/2027
5060078
07/2027
5060079
07/2027
5060080
07/2027
5080084
9/2027
Lot
Expiration Date
5080085
9/2027
5080086
9/2027
5090107
10/2027
5090108
10/2027
5090109
10/2027
5090113
10/2027
5090114
10/2027
5090115
10/2027
5090116
10/2027
5090117
10/2027
5090118
10/2027
5100039
11/2027
5100048
11/2027
The recalled products are packaged in white plastic bottles. The lot code is printed on the bottom of the bottles. The lot code is the middle seven digits of the code printed above the expiration date. Additionally, the affected product has expiration dates from 03/2027 to 11/2027. Please see the example packaging and lot code below.
No other Ambrosia Brands products are impacted by this recall.
To date, there have been 7 illnesses resulting in 3 hospitalizations across the United States due to Salmonella contamination, 3 of which may be linked to a single product. At this time, the FDA and CDC have reported that the outbreak may be linked to Rosabella Moringa Capsules.
We continue to diligently investigate, in collaboration with FDA, this possible link of the Salmonella outbreak to Rosebella Moringa Capsules. We have discontinued use and purchase of all raw moringa leaf powder from the raw material supplier of the above referenced lots.
Customers who have purchased the lots above are asked to dispose of it immediately, do not eat, sell, or serve the product.
True Metrix Blood Glucose Monitoring Systems
****************RECALL****************
Necessary Information:
Web Link- https://www.fda.gov/.../trividia-health-inc-initiates...
See original announcement for examples of labels
Company Announcement:
(FT. LAUDERDALE, FL) – February 6, 2026 – Trividia Health, Inc., announced today that it is initiating a labeling correction which requires a modification of the Owner’s Booklets/System Instructions for Use for all TRUE METRIX, TRUE METRIX AIR, TRUE METRIX GO, and TRUE METRIX PRO Blood Glucose Monitoring Systems (collectively, the “Products”) distributed in the United States, United Kingdom, Mexico, Australia, and the Caribbean.
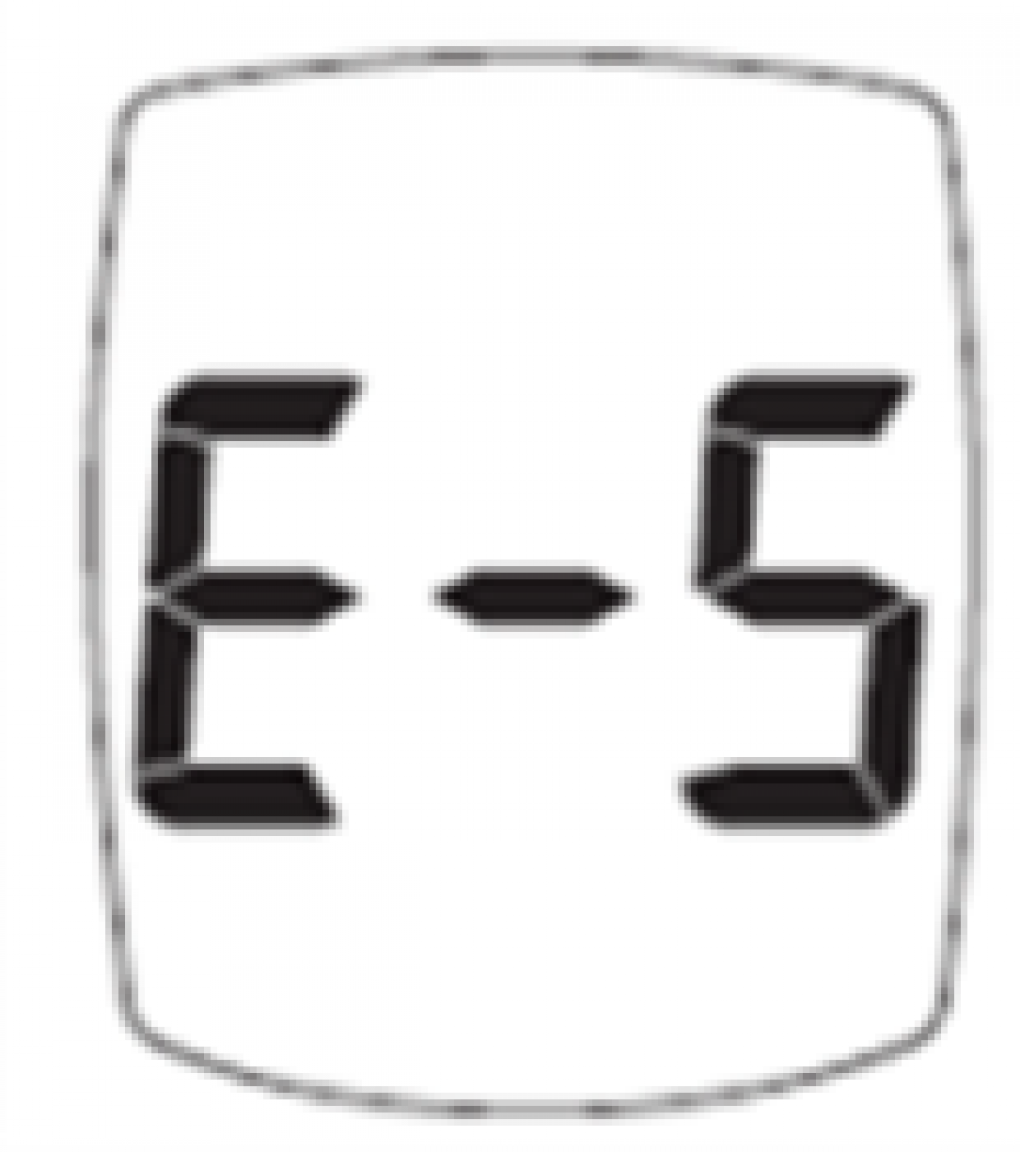
Trividia is updating the E-5 Error Code in the “Messages” section of the Owner’s Booklets/System Instructions for Use to emphasize that users must seek medical attention immediately if they receive an E-5 error code and are experiencing symptoms of high glucose.
The system displays an E-5 error code for a very high blood glucose event (> 600 mg/dL) or when there is a test strip error. As currently written, the instructions could potentially lead to a delay in treatment if the user does not seek medical attention immediately when they receive an E-5 error code and are experiencing symptoms of high glucose. A delay in treatment may result in serious adverse health consequences or death, especially for users with very high blood glucose levels.
Since August 2014, when TRUE METRIX was launched globally, there have been 114 reported serious injuries, and one (1) death associated with the E-5 Error Code.
UPDATED E-5 INSTRUCTIONS for TRUE METRIX, TRUE METRIX AIR, and TRUE METRIX GO:
Display Reason Action
SEE IMAGE Very high blood WARNING!! Retest with a new
BELOW glucose result (higher test strip. If the error persists
than 600 mg/dl). or and you have symptoms such
test strip error. as fatigue, excess urination,
thirst or blurry vision, seek
medical attention immediately.
If you are not experiencing
symptoms, retest with a new
test strip. If the error persists,
call 1-800-803-6025, Monday-
Friday, 8AM-8PM EST for
assistance.
UPDATED E-5 INSTRUCTIONS for TRUE METRIX PRO:
Display Reason Action
SEE IMAGE Very high blood WARNING!! Retest with a
BELOW glucose result (higher test strip. If the error persists
than 600 mg/dk). Or and you have symptoms such
test strip error. as fatigue, excess urination,
thirst, or blurry vision, seek
medical attention immediately.
If you are not experiencing
symptoms, retest with a new
test striip. If the error persists,
call 1-800-803-6025, Monday -
Friday, 8AM-8PM EST for
assistance.
If you have any questions relating to the Owner’s Booklets/System Instructions for Use update, please call Trividia Health Customer Care Department toll-free at 1-888-835-2723 Monday - Friday 8AM-8PM EST (excluding holidays) or e-mail trividia0126CC@trividiahealth.com or visit www.trividiahealth.com/E-5productnoticeExternal Link Disclaimer.
The correction affects all TRUE METRIX branded Blood Glucose Meters distributed in the United States, United Kingdom, Mexico, Australia and the Caribbean. This includes our cobranded products sold under store or distribution partner names. Please refer to the Product Notice located at www.trividiahealth.com/E-5productnoticeExternal Link Disclaimer for more information on the list of co-brand partners and affected product labeling.
The company is sending the Product Notice to impacted customers with instructions on what to do, to post the notice where products are stored/sold, and to forward the notice to device users, if possible. These customers include pharmacies, mail order companies, and distributors where the TRUE METRIX® meters are sold. Please refer to the Product Notice located at www.trividiahealth.com/E-5productnoticeExternal Link Disclaimer for more information.
You may continue to use the TRUE METRIX® Products. Products are not to be returned or replaced. This correction does not require removal of the Products from where they are used or sold. This labeling correction impacts the Owner’s Booklets/System Instructions for Use that accompany the meters at purchase, as well as the online labeling and help guides located on Trividia Health’s website. Trividia Health will notify users of additional mitigation strategies as needed.
Trividia Health has notified the U.S. Food and Drug Administration (FDA) of this action.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
Complete and submit the report Online www.fda.gov/medwatch/report.htm
Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
Patient safety is our top priority, and we apologize for any inconvenience this correction may cause you.
About Trividia Health
Trividia Health, Inc., is a global health and wellness company based in Fort Lauderdale, Florida and a leading developer, manufacturer and marketer of advanced performance products for people with diabetes. With products sold under TRUE and store brand labels, the company is the exclusive partner and supplier of affordable, high-quality blood glucose monitoring and health and wellness solutions for the world’s leading retail pharmacies, distributors and mail service providers. For more information, please visit: www.TrividiaHealth.comExternal Link Disclaimer.
Arrowroot biscuits recall
Necessary Information:
See original announcement for examples of labels
Company Announcement
ARLINGTON, VA., January 26, 2026 — Gerber Products Company is initiating a voluntary recall of limited batches of Gerber® Arrowroot Biscuits out of an abundance of caution due to the potential presence of soft plastic and/or paper pieces that should not be consumed. The material comes from an arrowroot flour supplier who initiated a recall. We are no longer working with the flour supplier.
This recall is isolated to limited batches of Gerber® Arrowroot Biscuits 5.5oz products produced between July 2025 and September 2025. In the U.S., this recall is nationwide.
No other Gerber® products are impacted by this recall.
Batch codes can be identified on the back of the product packaging. Please utilize reference images below and look for the 10-digit batch code prior to the best before date.
PRODUCT PACKAGING BATCH CODE BEST BEFORE DATE
5198565504 Oct. 16, 2026
5202565504 Oct. 20, 2026
5203565504 Oct. 21, 2026
5204565504 Oct. 22, 2026
5205565504 Oct. 23, 2026
5209565504 Oct. 27, 2026
5210565504 Oct. 28, 2026
5211565504 Oct. 29, 2026
5233565504 Nov. 20, 2026
5238565504 Nov. 25, 2026
5239565504 Nov. 26, 2026
5239565505 Nov. 26, 2026
5240565505 Nov. 27, 2026
5245565505 Dec. 2, 2026
5246565505 Dec. 3, 2026
5251565505 Dec. 8, 2026
5252565505 Dec. 9, 2026
5253565505 Dec.10, 2026
5254565505 Dec. 11, 2026
5258565505 Dec. 15, 2026
5259565505 Dec. 16, 2026
Consumers who may have purchased impacted Gerber® Arrowroot Biscuits should not feed this product to their child and can return the product to the retailer where it was purchased to receive a refund.
While no illnesses or injuries have been reported, we are acting out of an abundance of caution following a recall from the supplier. We are working closely with the U.S. Food & Drug Administration (FDA) and will cooperate fully throughout their review.
The quality, safety and integrity of our products remain our highest priority, and we take this responsibility seriously. We sincerely apologize for any concerns or inconvenience this action causes for parents, caregivers and retail customers.
*************MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES RECALL ALERT***************************
DATE: 1/30/2026
CLASS: Class I High
·
Necessary Information:
· Web Links
· Recall: https://www.fda.gov/.../why-not-natural-houston-texas...
· FDA Outbreak Investigation: https://www.fda.gov/.../outbreak-investigation-salmonella...
· CDC Outbreak Investigation: https://www.cdc.gov/.../supergreenssup.../investigation.html
· See original announcement for examples of labels
Company Announcement
Why Not Natural, Houston, Texas, is voluntarily recalling its Why Not Natural Organic Moringa - Green Superfood because they may be contaminated with Salmonella, an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Healthy persons infected with Salmonella often experience fever, diarrhea (which may be bloody), nausea, vomiting and abdominal pain. In rare circumstances, infection with Salmonella can result in the organism getting into the bloodstream and producing more severe illnesses such as arterial infections (i.e., infected aneurysms), endocarditis and arthritis.
The Moringa Capsules were distributed nationwide through mail orders on Why Not Natural’s e-commerce site and third-party on-line retailers including Amazon.comExternal Link Disclaimer from July 2025 to January 2026.
The product is packaged in a 120 capsule bottle marked with lot # A25G051 on the bottom and with an expiration date of 07/2028 stamped on the bottom.
The potential for contamination was noted by FDA as part of ongoing outbreak investigation of Salmonella in other products containing moringa powder. Distribution and production of the product has been suspended while FDA and Why Not Natural continue their investigation and Why Not Natural has further notified its customers of the recall.
Live it Up Super Greens Recall. (See Pictures Below)
Necessary Information:
Web Links
FDA Outbreak Investigation: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-salmonella-dietary-supplement-january-2026
CDC Outbreak Investigation: https://www.cdc.gov/salmonella/outbreaks/supergreenssupplementpowders-1-26/index.html
See original announcement for examples of labels
Company Announcement
Superfoods Inc. DBA as Live it Up of New York, New York is recalling all Live it Up Super Greens, including both Original and Wild Berry flavors, with lots beginning with the letter “A” and all stick pack products due to possible contamination with Salmonella, an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Healthy persons infected with Salmonella often experience fever, diarrhea (which may be bloody), nausea, vomiting and abdominal pain. In rare circumstances, infection with Salmonella can result in the organism getting into the bloodstream and producing more severe illnesses such as arterial infections (i.e., infected aneurysms), endocarditis and arthritis.
The recalled product was sold across the United States online through our direct-to-consumer website since January, 2025 and on Amazon.com since July 30, 2025.
The recall includes the following products:
Live it Up Super Greens, NET WT 8.5 oz (240g) with UPC 860013190804.
Live it Up Super Greens, 30 – 0.28oz (8g) sticks, NET WT. 8.47 oz (240g) with UPC 850077468063
Live it Up Super Greens, Wild Berry, NET WT 8.5OZ (240g), with UPC 860013190811
Live it Up Super Greens, Wild Berry, 30 – 0.32oz (9g) Sticks, NET WT. 9.52oz (270g), with UPC 850077468070
The recalled products are packaged in green pouches. The lot code is printed on the bottom back of the packaging in black and begins with the letter “A” or the number “3” for a stick pack product. Additionally, the affected product has expiration dates from 08/2026 to 01/2028. Please see the example packaging and lot code below.
To date, there have been 45 illnesses and 12 hospitalizations across the United States due to salmonella contamination linked to a single product. At this time, the FDA and CDC have reported that the outbreak may be linked to Live it Up Super Greens. No other Live it Up products are involved in this recall at this time. Production of the product has been suspended while FDA and the company continue their investigation as to the source of the problem.
Customers who have purchased the affected product are asked to dispose of it immediately, do not eat, sell, or serve the product. Customers may request a refund by contacting Live it Up at recall@artofenso.com and provide your name, order number and a photo of the product with lot codes beginning with “A” and stick pack products.
Live it Up takes food safety extremely seriously. We apologize for the inconvenience and concern this recall may cause our customers. Our company is committed to ensuring the quality of our products and the well-being of our consumers.
FreeStyle Libre 3 and Libre 3 Plus sensors
Necessary Information:
See original announcement for examples of labels
Company Announcement:
Medical device correction impacts a subset of FreeStyle Libre 3 and Libre 3 Plus sensors
Consumers can visit www.FreeStyleCheck.comExternal Link Disclaimer to see if their sensors are affected and to get a replacement at no charge
Abbott has identified and resolved the manufacturing issue related to this device action
No other Libre family sensors, readers or apps are impacted
ABBOTT PARK, Ill., Nov. 24, 2025 — Abbott has initiated a medical device correction for certain FreeStyle Libre 3 and FreeStyle Libre 3 Plus sensors in the United States after internal testing determined that some sensors may provide incorrect low glucose readings.
If undetected, incorrect low glucose readings over an extended period may lead to incorrect treatment decisions for people living with diabetes, such as excessive carbohydrate intake or skipping or delaying insulin doses. These decisions may pose serious health risks, including potential injury or death, or other less serious complications.
Abbott has identified and resolved the cause of the issue, which relates to one production line among several that make Libre 3 and Libre 3 Plus sensors. The company continues to produce Libre 3 and Libre 3 Plus sensors to fulfill replacement and new orders and does not expect significant supply disruptions.
This action involves approximately 3 million Libre 3 and Libre 3 Plus sensors in the U.S. from that production line, about half of which are estimated to have expired or been used. Globally, Abbott has received reports of 736 severe adverse events (57 in the U.S.) and seven deaths (none in the U.S.) potentially associated with this issue.
Important Instructions for People Using Libre 3 or Libre 3 Plus Sensors
Consumers should visit www.FreeStyleCheck.comExternal Link Disclaimer to confirm whether their sensor is potentially affected by this medical device correction. Abbott will replace any potentially affected sensors at no charge. Detailed instructions on how to check sensors and request a replacement are available on www.FreeStyleCheck.comExternal Link Disclaimer.
If consumers are currently wearing or have a sensor that has been confirmed as potentially impacted on www.FreeStyleCheck.comExternal Link Disclaimer or by a customer service representative, they should immediately discontinue use and dispose of it.
Consumers should use a blood glucose meter or the built-in meter in the FreeStyle Libre 3 reader to make treatment decisions when sensor readings don’t match symptoms or expectations.
To learn more or get help with questions, visit www.FreeStyleCheck.comExternal Link Disclaimer or call Abbott’s customer service at 1-833-815-4273, available seven days a week from 8 a.m. to 8 p.m. Eastern Time. Agents are available 24/7 through live chat at https://www.freestyle.abbott/us-en/support/contact-us.htmlExternal Link Disclaimer.
FreeStyle Libre 3 readers and mobile apps are not impacted. Additionally, no other Libre products (FreeStyle Libre 14 day, FreeStyle Libre 2, FreeStyle Libre 2 Plus, or Libre Pro sensors) or Abbott biowearables are impacted.
Abbott is issuing this medical device correction in other affected countries where Libre 3 and Libre 3 Plus sensors are distributed. Consumers in other countries can also visit www.FreeStyleCheck.comExternal Link Disclaimer for more information.
About Abbott:
Abbott is a global healthcare leader that helps people live more fully at all stages of life. Our portfolio of life-changing technologies spans the spectrum of healthcare, with leading businesses and products in diagnostics, medical devices, nutritionals and branded generic medicines. Our 114,000 colleagues serve people in more than 160 countries. Connect with us at Abbott.com and on LinkedInExternal Link Disclaimer, FacebookExternal Link Disclaimer, InstagramExternal Link Disclaimer, X and YouTubeExternal Link Disclaimer.
Homer Pool drain cover
Necessary Information:
See original announcement for examples of labels
Company Announcement:
Recall Details
Description:
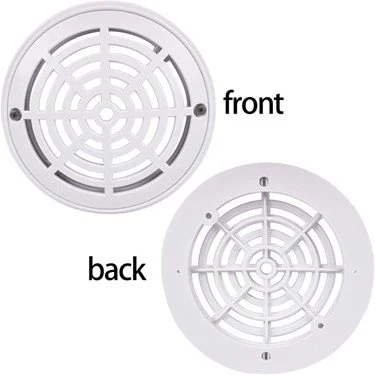
This recall involves TopHomer-branded 8-inch pool drain covers that are sold for use in swimming pools. The top grate is 7.2 inches in diameter, and the bottom mounting plate is 8 inches in diameter. They are white and made of ABS plastic. “Listed suction fitting,” “SP-1053-B,” and “88D9 148 GPM” are printed on the center of the grate. “Use screws to keep grate secured to dram (drain) at all times” is printed on the outer ring of the grate.
Remedy:
Pool owners, pool operators and consumers should immediately stop using pools with the recalled pool drain covers. To receive a refund, consumers should remove the drain cover from the pool, take a photo of the removed cover, and send it to the recalling firm by email at Tophomer.recall@outlook.com. Ensure all pools and spas have VGBA-compliant drain covers and teach children to stay away from drains.
Incidents/Injuries:
None reported
Sold At:
Amazon.com from August 2024 through October 2025 for between $15 and $26.
Retailer:
Guangzhoushuoshenghaoyikejiyouxiangongsi, dba TopHomerUS, of China
Manufactured In:
China
Recall number:
26-154
tomato basil soup recall
Necessary Information:
See original announcement for examples of labels
Company Announcement:
Lil’ Turtles is recalling all lots of its Grandma Belle’s Tomato Basil Soup for misbranding due to the milk allergen not being declared on the label. The Grandma Belle’s Tomato Basil Soup was packaged in 17 oz glass jars.
Product was available from 9-23-22 to 12-3-25 in the following retail stores:
Baltic Meats, Baltic, OH 43804
Trailside Deli, Millersburg, OH 44654
Randles Cheese, Millersburg, OH 44654
Wesley Miller, Sugarcreek, OH 44681
Bambi Farm Market, Orient, OH 74337
Baker Florist, Dover, OH 44622
Jungle Jim, Cincinnati, OH 45254
Becks Market, Greenville, OH 45331
Indian Trail Country Market, Elizabethville, PA 17023
Burger Farms, Drums, PA 18222
Paul’s Country Market, Waynesboro, PA 17268
The Olde Bat Factory, Hancock, NY 13783
Shadyview Farm, Rushville, NY 14544
Beef and Butter Company, Rochester, NY 14623
Hymers Farm Market, Delhi, NY
Troyers Country Store, Canastota, NY 13032
Creekside Pantry, London, KY 40741
Cherry Grove Market, Browerville, MN 56438
Applecreek Willard, Willard, UT 84340
Amish Cheesehouse, Chouteau, OK 74337
Martins House Country Store, Davis, OK 73030
Jet Produce, Leavenworth, KS 66048
45 Flea Market, Meridian, MS 39305
Countryside Variety, Buncombe, IL 62912
Dented Can, Goshen, IN 46526
Sommher’s Bakery, Gosport, IN 47433
Sunnyside Market, El Dorado, MO 64744
Hecks Farm Market, Arena, WI 53503
Golden Delight Bakery, Kalona, IA 52247
The issue was discovered during a routine inspection conducted by the Ohio Department of Agriculture. There have been no reports of illness involving the product addressed in this recall, however, people who have an allergy or severe sensitivity to milk run the risk of a serious allergic reaction if they consume this product.
Consumers with a milk allergy or sensitivity who have purchased the affected product should contact Lil’ Turtles for a replacement pack. Consumers with questions regarding this recall can contact Josh Coblentz at 330-897-6400.
RITZ Peanut Butter Cracker Sandwiches
******************RECALL***************
Necessary Information:
Web Link- https://www.fda.gov/.../mondelez-global-llc-conducts...
See original announcement for examples of labels
Company Announcement:
EAST HANOVER, N.J., Nov. 28, 2025 (GLOBE NEWSWIRE) -- Mondelēz Global LLC announced today a voluntary recall of 70 cases of RITZ Peanut Butter Cracker Sandwiches and sold in the following U.S. states: New York, New Jersey, Pennsylvania, Georgia, Arkansas, Missouri, Oklahoma, and Alabama.
This recall is limited to 1 SKU and 2 Code Dates previously recalled in July. This action is not an expansion of that prior recall and is being conducted out of an abundance of caution.
The affected cartons include individually wrapped packs that may be incorrectly labeled as Cheese variety even though the product may be a Peanut Butter variety. People who have an allergy or severe sensitivity to peanuts may risk serious or life-threatening allergic reactions by consuming this product.
All outer cartons affected are labeled correctly and provide an allergen advisory statement indicating that the product “contains peanuts.”
This recall is exclusively for the RITZ Peanut Butter Cracker Sandwich cartons with Best When Used By Dates listed in the grid below, available at a limited number of retail stores nationwide. No other RITZ products or Mondelēz Global LLC products are included in, or affected by, this recall.
Product Description:
27.6 oz. RITZ Peanut Butter Cracker Sandwiches
- 20 Count (20 x 1.38-oz. 6-pack carton)
Retail UPC:
44000 07584 2
Best When Used By Dates:
8 JAN 26
15 Jan 26
“AE” Plant Code Only
(located on top of package)
Product Images:
See Image Below
Cartons containing only RITZ Cheese Cracker Sandwiches are not affected. In addition, cartons containing either RITZ Peanut Butter Cracker Sandwiches or RITZ Filled Cracker Sandwich Variety Pack with different Best When Used By Dates and Plant Codes than those listed in the above grid are not affected by this recall.
There have been no reports of injury or illness reported to Mondelēz Global LLC to date related to this product, and we are issuing this recall out of an abundance of caution.
The recall was initiated after Mondelēz Global LLC discovered that 70 cases were inadvertently shipped to a limited number of retailers in eight states. Corrective actions are being taken.
Consumers who have a peanut allergy should not eat these products and should discard any product identified in the grid above. Consumers can contact the company at 1-844-366-1171 Monday–Friday, 9 am to 6 pm ET.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
About Mondelēz International
Mondelēz International, Inc. (Nasdaq: MDLZExternal Link Disclaimer) empowers people to snack right in over 150 countries around the world. With 2024 net revenues of approximately $36.4 billion, MDLZ is leading the future of snacking with iconic global and local brands such as OREO, RITZ, LU, CLIF BAR and TATE’S BAKE SHOP biscuits and baked snacks, as well as CADBURY DAIRY MILK, MILKA and TOBLERONE chocolate. Mondelēz International is a proud member of the Standard and Poor’s 500, Nasdaq 100 and Dow Jones Sustainability Index.
Visit www.mondelezinternational.comExternal Link Disclaimer or follow the company on X at www.x.com/MDLZExternal Link Disclaimer.
Contacts:
Jane Corcoran 847-943-5678
news@mdlz.comExternal Link Disclaimer
Ambriola Company Cheese Recall
Necessary Information:
See original announcement for examples of labels
Company Announcement:
[West Caldwell, NJ] – November 25, 2025 – The Ambriola Company is recalling select cheese products after routine testing confirmed the presence of Listeria monocytogenes, an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Although healthy individuals may suffer only short-term symptoms such as high fever, severe headache, stiffness, nausea, abdominal pain and diarrhea, Listeria infection can cause miscarriages and stillbirths among pregnant women. Out of an abundance of caution, the Company is also recalling additional cheese products processed at the same facility in West Caldwell, New Jersey.
No illnesses have been reported to date. That said, customers who have symptoms of listeria infection should contact their healthcare provider.
The affected products were distributed to retail stores and distributors nationwide between November 3, 2025 and November 20, 2025 and include:
Retail Product (Exact Weight)
Expiration Dates
Locatelli Pecorino Romano Grated 4 oz. cup
05/03/26, 05/10/26, 05/17/26
Locatelli Grated Pecorino Romano 8 oz. cup
04/06/26, 04/11/26, 04/12/26, 04/15/26, 04/17/26
05/05/26, 05/06/26, 05/07/26, 05/10/26
05/12/26, 05/14/26, 05/17/26
Boar’s Head Grated Pecorino Romano 6 oz. cup
03/04/26, 03/12/2026
Member’s Mark Pecorino Romano 1.5 lb. Bag
03/25/26, 03/30/26, 04/05/26
Grated Product Sold by the Pound
Expiration Dates
Locatelli Grated Pecorino Romano
03/04/26, 03/06/26, 03/11/26, 03/13/26
Ambriola Grated Pecorino Romano
02/28/26, 03/04/26, 03/11/26
Pinna Grated Pecorino Romano
03/11/26
Boar’s Head Pecorino Romano Grated bag
03/03/26, 03/12/26
No other Ambriola, Locatelli, Member’s Mark, Pinna, or Boar’s Head products are included in the recall.
Customers who purchased the affected products should not consume them and either dispose of them or return them to the place of purchase for a full refund. For more information, contact Ambriola at 1-800-962-8224 from Monday through Friday from 9:00am – 4:00 pm ET.
“We take food safety very seriously and immediately alerted stores and distributors to remove the affected products from shelves,” said Phil Marfuggi, chief executive officer. “We are working closely with the FDA and continuing to test our products and facilities to fully understand the situation.”
Ambriola has suspended production and distribution of affected products as the Company conducts a thorough review of all sanitation and food safety procedures.
Choceur branded Holiday Bark Recall
Necessary Information:
See original announcement for examples of labels
Company Announcement:
Silvestri Sweets Inc. of Geneva, IL is voluntarily recalling its 5-ounce bags of Choceur branded Holiday Barks because they may contain undeclared allergens.
Choceur branded Cookie Butter Holiday Bark may contain undeclared pecans. People who have allergies to pecans run the risk of serious or life-threatening allergic reaction if they consume these products.
Choceur branded Pecan, Cranberry & Cinnamon Holiday Bark may contain undeclared wheat. People who have allergies to wheat run the risk of serious or life-threatening allergic reaction if they consume these products.
The recalled items were distributed nationwide through Aldi grocery stores.
Cookie Butter Holiday Bark is packed in 5 oz. Choceur branded stand up pouch bags with the lot # 29225 and best by date on 05/2026, which are printed on the back of each bag.
Pecan, Cranberry & Cinnamon Holiday Bark is packed in 5 oz. Choceur branded stand up pouch bags with the lot # 29225 and best by date on 08/2026, which are printed on the back of each bag.
No illnesses have been reported to date in connection with this problem.
The recall was initiated after it was discovered that the Pecan, Cranberry & Cinnamon Holiday Bark had been packaged in Cookie Butter Holiday Bark packages resulting in undeclared pecan, and that the Cookie Butter Holiday Bark has been packaged in Pecan, Cranberry & Cinnamon Holiday Bark packages resulting in undeclared wheat. Subsequent investigation indicates the problem may have been caused by a temporary breakdown in the company’s production and packaging process.
Consumers who have purchased the recalled products are urged to discard the product.
Consumers with questions may contact Silvestri Sweets at 1-630-232-2500 – M-F 8:30am – 4:30pm CST.
Prime Food brand Lava Bun
****RECALL****
Necessary Information:
• Web Link- https://www.fda.gov/.../prime-food-processing-llc-issues...
• See original announcement for examples of labels
Company Announcement:
Prime Food Processing LLC of Brooklyn, NY is voluntarily recalling 2,243 cases of two dessert bun varieties because the packaging does not declare milk in the “Contains” allergen statement as required by the U.S. Food and Drug Administration (FDA). The milk in these products is derived from unsalted butter listed in the ingredient statement. People who have an allergy or severe sensitivity to milk risk a serious or life-threatening allergic reaction if they consume these products.
The recalled products were distributed to Asian grocery stores between April 2, 2025 and November 14, 2025 in the following states:
AL, AZ, CA, CO, CT, FL, GA, HI, IL, IN, KS, LA, MA, MD, ME, MI, MO, MS, NC, NE, NJ, NY, OH, OK, OR, PA, RI, TN, TX, UT, VA, WA, WI
Recalled Products include:
Prime Food brand Lava Bun with Salted Egg Yolk with UPC #97903705873- 24 oz Item #PD4188 – This product is packaged in an orange pouch approximately 10.25"W × 11.25"H Lot Code Range: 25092-25318. Expiration Range: 07/26-03/27
Prime Food brand Lava Bun with Green Tea Flavor UPC #97803705883- 24 oz Item #PD4198 – This product is packaged in a bright lime green pouch approximately 10.25"W × 11.25"H Lot Code Range: 25092-25318. Expiration Range: 07/26-03/27
No illnesses or allergic reactions have been reported to date.
The issue was identified during an internal product review. The recall was initiated after it was discovered that packaging did not declare milk in the allergen statement. Subsequent investigation indicates the problem was caused by a temporary breakdown in the company’s label review process.
Consumers who purchased these products should return them to the place of purchase with the uneaten buns and packaging for a full refund.
Consumers with questions may contact Prime Food Processing LLC at 718-963-2323, Monday–Friday, 9:00 AM to 5:00 PM EST.
Organic Moringa Leaf Powder
***********RECALL*******************
Brooklyn, NY – Food To Live, of Brooklyn, NY, is recalling its packaged Organic Moringa Leaf Powder (dried Moringa oleifera) and its Organic Supergreens Powder Mix distributed under the Food To Live brand that are in the following sizes:
ORGANIC MORINGA LEAF POWDER, in 8 ounce, 1 pound, 2 pound, 4 pound, 8 pound, 16 pound, and 44 pound bags.
ORGANIC SUPERGREENS POWDER MIX in 8 ounce, 1 pound, 1.5 pound, 3 pound, 6 pound, and 12 pound bags.
These products have the potential to be contaminated with Salmonella. Salmonella is an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Healthy persons infected with Salmonella often experience fever, diarrhea (which may be bloody), nausea, vomiting, and abdominal pain. In rare circumstances, infection with Salmonella can result in the organism entering the bloodstream and producing more severe illnesses such as arterial infections, endocarditis, and arthritis.
The recalled Organic Moringa Powder was distributed nationwide through both retail and wholesale channels. It was sold directly from Food To Live’s website and shipped to customers across the United States. The product was also available on third-party e-commerce platforms, including Amazon.com, Walmart.com, Target, Etsy, and eBay. In addition, bulk quantities from the affected lot were sold to food manufacturers and other businesses through Food To Live’s wholesale division.
“Organic Moringa Leaf Powder” and “Organic Supergreens Powder Mix” are packaged in plastic stand-up pouches of various sizes (ranging from 8 ounces to 44 pounds). Only packages with lot codes starting with “SO-69006” and ending with “SO-72558” (printed directly onto the back of the bag) are affected. No other Food To Live products and lots are involved in this recall.
This recall was initiated after the FDA notified Food To Live that a specific supplier lot of organic moringa powder tested positive for Salmonella and has been linked to an outbreak of salmonellosis. Multiple illnesses have been reported in connection with this contaminated lot across several states (FDA and CDC are investigating these cases). Food To Live has immediately ceased distribution of this product.
We have suspended production and distribution of Organic Moringa Leaf Powder while we and the FDA continue to investigate the source and extent of the problem. Food To Live is working closely with the FDA to ensure all potentially unsafe product is removed from commerce.
Consumers who have purchased Food To Live Organic Moringa Powder or Food To Live Organic Supergreens Powder Mix with the lot codes mentioned above should not consume it. If you have the product, we urge you to dispose of it immediately or return it to the place of purchase for a full refund. For online purchases, you may contact Food To Live directly for a refund or replacement. Consumers with questions can call Food To Live at (718) 717-1029 during business hours (8:00 AM EST to 5:00 PM EST, Monday through Friday, or email recall@foodtolive.com for assistance. Media inquiries or requests for more information should be directed to the contact person listed above.
Food To Live takes food safety extremely seriously. We apologize for the inconvenience and concern this recall may cause our customers. Our company is committed to ensuring the quality of our products and the well-being of our consumers.
byheart whole nutrition infant formula
****RECALL****
Necessary Information:
• Web Link- https://www.fda.gov/.../response-broader-fda...
• See original announcement for examples of labels
Company Announcement:
[New York, NY] – ByHeartExternal Link Disclaimer, a next-generation baby nutrition company, announced today that, out of an abundance of caution, it has chosen to voluntarily recall two batches of ByHeart Whole Nutrition Infant Formula following notification from the U.S. Food and Drug Administration (FDA) of a broader ongoing investigation into a recent outbreak of infant botulism.
Infant botulism is a rare but potentially fatal illness that presents a serious threat to the health of infants which occurs when Clostridium botulinum spores are ingested and colonize the intestinal tract, producing botulinum neurotoxins in the immature gut of infants. Affected infants can present with some or all of the following signs and symptoms: constipation, poor feeding, ptosis (drooping eyelid), sluggish pupils, low muscle tone, difficulty sucking and swallowing, weak or altered cry, generalized weakness, respiratory difficulty, and possibly respiratory arrest.
• The FDA has an ongoing investigation of infant botulism among babies in the U.S.
• The FDA has not identified a direct link between any infant formula and these cases and there is no historical precedent of infant formula causing infant botulism.
• ByHeart is taking the proactive step to remove any potential risk from the market and ensure the highest level of safety for infants.
ByHeart was notified by the FDA on November 7, 2025 of an estimated 83 cases of infant botulism that were reported nationwide since August 2025. Of these, the FDA also noted that 13 infants received ByHeart formula at some point. The FDA has not identified a direct link between any infant formula and these cases and there is no historical precedent of infant formula causing infant botulism. Botulism is extremely uncommon in dairy products or infant formula, and is naturally occurring in environmental sources like soil, select vegetables, and dust.
ByHeart adheres to the highest standards of Global and U.S. recommendations for product testing and safety. No ByHeart product has tested positive for any contaminants.
“The safety and well-being of every infant who uses our formula is our absolute highest priority,” said Mia Funt, Co-Founder and President. “We take any potential safety concern extremely seriously, and act quickly to protect families. As parents ourselves, we understand the concern this news may raise. This voluntary recall is out of an abundance of caution and comes from our ongoing commitment to transparency and safety for babies and their parents. While no testing by ByHeart or regulatory agencies has confirmed the presence of Clostridium botulinum spores or toxin in any ByHeart product, we are taking this proactive step to remove any potential risk from the market and ensure the highest level of safety for infants.”
Product Details for Voluntary Recall:
The voluntary recall applies to the following two batches of ByHeart infant formula:
• Batch Code: 251261P2, Use by: 01 Dec 2026
• Batch Code: 251131P2, Use by: 01 Dec 2026
• UPC: 5004496800
• Product Image : See below
•
The batch code and use by can be found on the bottom of the can.
No other ByHeart batches are impacted by this voluntary recall.
What Consumers Should Do:
Consumers who have purchased ByHeart infant formula from the identified batch codes should immediately discontinue use and dispose of the product. If you’ve discarded any formula from the following batches, ByHeart will replace those cans at no cost.
If your infant is experiencing symptoms related to infant botulism, contact your health care provider immediately. To report an illness or adverse event, you can:
• Call an FDA Consumer Complaint Coordinator if you wish to speak directly to a person about your problem.
• Complete an electronic Voluntary MedWatch form online.
• Complete a paper Voluntary MedWatch form that can be mailed to the FDA.
If any parents have questions please contact our experts at hello@byheart.com. For more information visit byheart.comExternal Link Disclaimer or call 1 (833) 429 - 4327 We are available 24/7.
FDA Outbreak Advisory
Häagen-Dazs Chocolate Dark Chocolate Mini Bars recal
***RECALL***
Necessary Information:
• Web Link- https://www.fda.gov/.../dreyers-grand-ice-cream-inc...
• See original announcement for examples of labels
Company Announcement:
November 3, 2025, Dreyer’s Grand Ice Cream, Inc. is voluntarily recalling a limited number of its Häagen-Dazs Chocolate Dark Chocolate Mini Bars that may contain undeclared wheat. Those with an allergy or severe sensitivity to wheat run the risk of serious or life-threatening allergic reaction if they consume these products.
The affected product is Häagen-Dazs Chocolate Dark Chocolate Mini Bars in the 6-count package with batch code LLA519501 and a Best By date of January 31, 2027.
The affected product was shipped to two retail customers, Kroger and Giant Eagle, in the following states:
• Kroger: (AL, AK, AZ, AR, CA, CO, GA, ID, IL, IN, KS, KY, MI, MS, MO, MT, NE, NV, NM, OH, OR, SC, TN, UT, VA, WA, WV, WI, WY)
• Giant Eagle: (IN, MD, OH, PA, WV)
Batch codes can be identified on the product packaging. Please use the reference images below and look for the batch code LLA519501 under “Best By 31 JAN 2027.”
No other Häagen-Dazs products or other batches of Häagen-Dazs Chocolate Dark Chocolate Mini Bars are affected by this recall.
No illnesses or injuries have been reported to date. We are recalling this product because it may contain products that contain wheat in packaging that does not reveal the presence of wheat on the label. Although our investigation is ongoing, we believe products containing wheat were repacked into the incorrect packaging at the beginning of a production run.
Consumers with a wheat allergy or sensitivity who have purchased the affected product are urged not to consume the product and instead dispose of it or return it to their place of purchase for a full refund.
The safety, quality, and integrity of our products remain our number one priority. We sincerely apologize for any inconvenience this action represents to both our consumers and retail customers.
We are working with the U.S. Food and Drug Administration (FDA) on this voluntary recall and will cooperate with them fully.
alifornia-grown conventional yellow and white peaches Recall
Necessary Information:
• Web Link- https://www.fda.gov/.../moonlight-companies-voluntarily...
• See original announcement for examples of labels
•
Company Announcement:
October 29, 2025 – Moonlight Companies is voluntarily recalling California-grown conventional yellow and white peaches because they have the potential to be contaminated with Listeria monocytogenes, an organism that can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Although healthy individuals may suffer only short-term symptoms such as high fever, severe headache, stiffness, nausea, abdominal pain, and diarrhea. Listeria infection can cause miscarriages and stillbirths among pregnant women.
The recalled peaches were sold at retail stores nationwide between September 16, 2025 and October 29, 2025. The peaches were either sold as individual pieces of fruit bearing PLU stickers or as multi-packs. This recall does not include packages or PLU stickers with the words “Washington” and/or “Organic.” The recalled products are listed in the following summary table and images are below.
This recall is being conducted because Listeria monocytogenes was identified in the packing facility environment.
No illnesses have been reported to date.
More Ground Cinnamon Products Added to FDA Public Health Alert Due to Presence of Elevated Levels of Lead
More Ground Cinnamon Products Added to FDA Public Health Alert Due to Presence of Elevated Levels of Lead
What's New
October 30, 2025
The FDA is updating the alert below with additional products, DEVI-brand and BaiLiFeng-brand ground cinnamon, that contain elevated levels of lead. FDA collected samples of these products at a retail establishment. Exposure to these products may be unsafe. Please view the table and recommendations below for more details.
Original Public Health Alert
July 30, 2024
Audience
All consumers
All retailers
Product
Through product testing by state programs and confirmed by the FDA, the FDA has determined that the ground cinnamon products listed in the table below contain elevated levels of lead and that exposure to these products may be unsafe. The FDA is advising consumers to throw away and not to buy these ground cinnamon products.
Recommendations for Consumers
The FDA is advising consumers to stop using and dispose of the ground cinnamon products listed in the table below.
Consumers should not eat, sell, or serve ground cinnamon products listed in the table below and should discard them.
These products have a long shelf life. Consumers should check their homes and discard these products.
If there’s suspicion that someone has been exposed to elevated levels of lead, talk to your healthcare provider. Most people have no obvious immediate symptoms of lead exposure.
Consumers who have symptoms should contact their health care provider to report their symptoms and receive care. To report a complaint or adverse event (illness or serious allergic reaction), visit Industry and Consumer Assistance.
*Kenz Henz brand Grade AA Large Eggs Recall*
Whatever it is, the w** Kenz Henz brand Grade AA Large Eggs Recall**
Kenz Henz of Santa Fe, TX, is recalling its 12 count packages of "Grade AA Large Pasture Raised eggs" because they have the potential to be contaminated with Salmonella, an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Healthy persons infected with Salmonella often experience fever, diarrhea (which may be bloody), nausea, vomiting and abdominal pain. In rare circumstances, infection with Salmonella can result in the organism getting into the bloodstream and producing more severe illnesses such as arterial infections (i.e., infected aneurysms), endocarditis and arthritis.
The recalled "12 Count Pasture Raised eggs" in retail stores in Houston, TX.
The called Kenz Henz brand Grade AA Large Eggs are packaged in a 12 count carton marked with UPC code 86949400030, a Julian date of 241 (Aug 30) to 244 (Sep 2) and 246 (Sep 4) to 247 (Sep5) with a best by date of 10/11 to 10/14 and 10/16 to 10/17 stamped on the side.
No illnesses have been reported to date in connection with this problem.
Current update as of September 29, 2025: FDA initiated an inspection at Black Sheep Egg Company’s egg processing facility and collected environmental samples. Of the samples collected, 40 environmental samples were positive for Salmonella including seven different strains of Salmonella. Some of these strains are known to cause human illness. FDA does not have information available at this time to suggest that this firm is the source of an ongoing outbreak.
Consumers who purchased 12 count cartons of "Kenz Henz Grade AA pasture raised eggs" are urged to return them to the place of purchase for a full refund. Consumers with questions may contact the company at 1-(409)457-5934 Mon – Fri 9:00am – 4:00pm.
Company Contact Information
Consumers:
(409) 457-5934
The Black Sheep Egg Company first issued a voluntary recall of hundreds of thousands of cartons of eggs on Sept. 29 after FDA testing found seven different strains of salmonella in 40 samples taken from its Arkansas processing center. On Oct. 17, the FDA updated its advisory after Kenz Henz also issued a recall for its 12-count egg cartons.
Because Black Sheep distributes eggs to both wholesalers and retailers, some of the eggs may have been rebranded with different packaging by other companies. Some of the recalled items include large-scale deliveries of eggs sold to third parties, such as restaurants and retailers.
Which eggs were recalled?
· Black Sheep Egg Company 12-count cartons of free-range large grade A brown eggs with best by dates 8/22/2025-10/31/2025 and UPC 860010568507.
· Black Sheep Egg Company 18-count cartons of free-range large grade A brown eggs with best by dates 8/22/2025-10/31/2025 and UPC 860010568538.
· Kenz Henz 12-count cartons of grade AA large pasture-raised eggs with best by dates 10/11-10/14 and 10/16-10/17 and UPC 86949400030. Sold in retail stores in Houston, TX.
Recalled products from the Black Sheep Egg Company were also sold to retailers and other wholesale companies in Arkansas, Missouri, Texas, California and Indiana between July 9, 2025, and September 17, 2025, where they may have been repackaged. The FDA believes the eggs could have been distributed in more states and plans to update the recall list if more potentially contaminated products are identified.
What to do if you have recalled eggs
Consumers, restaurants, and retailers should not eat, sell or serve the recalled eggs, the FDA said. Customers can return the eggs to the place of purchase for a refund or dispose of them.
Fusia Asian Inspirations Veggie Spring Rolls
Necessary Information:
See original announcement for examples of labels
Company Announcement
October 8, 2025 – Tai Foong USA of Seattle, Washington is recalling a limited quantity of Fusia Asian Inspirations Veggie Spring Rolls, net wt. 10oz, because the product may contain shrimp, a known allergen, that is not declared on the label. People who have an allergy or severe sensitivity to shrimp or shellfish run the risk of a serious or life-threatening allergic reaction if they consume this product.
The affected Veggie Spring Rolls were distributed exclusively to ALDI stores nationwide and sold under the Fusia Asian Inspirations brand.
The product is a 10-ounce (283.5 g) retail package identified by UPC 4099100222258 and the Best Before date 05/17/2027 printed on the back panel of the retail box.
No other Fusia Asian Inspirations products are affected by this recall.
The recall was initiated after it was discovered that certain cases of Shrimp Spring Rolls may have been inadvertently packaged in boxes labeled as Vegetable Spring Rolls. The cause of this labeling error is being investigated. The recall is being conducted in cooperation with ALDI and the U.S. Food and Drug Administration.
At this time, no confirmed allergic reactions or illnesses have been reported related to this issue.
The Hillshire Brands Company Recalls Corn Dog and Sausage On A Stick Products Due To Possible Extraneous Matter Contamination
March 17, 2025, and September 26, 2025.
Necessary Information:
See original announcement for examples of labels
Company Announcement:
WASHINGTON, September 27, 2025 – The Hillshire Brands Company, a Haltom City, Tex. establishment, is recalling approximately 58,000,000 pounds of corn dog and sausage on a stick products that may be contaminated with extraneous material, specifically pieces of wood embedded in the batter, the U.S. Department of Agriculture’s Food Safety and Inspection Service (FSIS) announced today.
The corn dog and sausage on a stick products were packaged between March 17, 2025, and September 26, 2025. A list of the products subject to recall can be found here: [view product list]. The labels for the impacted products can be found here: [view labels].
The products subject to recall bear establishment number “EST-582” or “P-894” printed on the packaging. These items were sold online and shipped to retail and food service locations nationwide. They were also sold to school districts and Department of Defense facilities nationwide. While the products were distributed to schools, it resulted from commercial sales and not part of food provided by the USDA for the National School Lunch Program.
The problem was discovered after the establishment received multiple consumer complaints, five of which involved injuries. The Hillshire Brands Company conducted an investigation and determined that the wooden sticks entered the production process prior to product battering. FSIS has received no additional reports of injury from consumption of these products. Anyone concerned about an injury should contact a healthcare provider.
FSIS is concerned that some products may be in consumers’ refrigerators and freezers, along with school and institution refrigerators and freezers. Consumers, schools, and institutions who have purchased these products are urged not to consume them. These products should be thrown away or returned to the place of purchase.
FSIS routinely conducts recall effectiveness checks to verify recalling firms notify their customers of the recall and that steps are taken to make certain that the product is no longer available to consumers. When available, the retail distribution list(s) will be posted on the FSIS website at www.fsis.usda.gov/recalls.
Consumers with questions about the recall can contact Christina Self, The Hillshire Brands Company Associate Director of Customer Care, at 888-747-7611. Members of the media with questions about the recall can contact MaKenzie Taylor, The Hillshire Brands Company Communications Manager, at 810-391-6680.
Consumers with food safety questions can call the toll-free USDA Meat and Poultry Hotline at 888-MPHotline (888-674-6854) or send a question via email to MPHotline@usda.gov. For consumers that need to report a problem with a meat, poultry, or egg product, the online Electronic Consumer Complaint Monitoring System can be accessed 24 hours a day at https://foodcomplaint.fsis.usda.gov/eCCF/.
Necessary Information:
See original announcement for examples of labels
Company Announcement:
WASHINGTON, September 25, 2025 – The U.S. Department of Agriculture’s Food Safety and Inspection Service (FSIS) is issuing a public health alert for ready-to-eat meals containing a Food and Drug Administration (FDA) regulated pre-cooked pasta that may be contaminated with Listeria monocytogenes (Lm). FSIS expects more updates as this investigation continues. As more information becomes available, FSIS will update this public health alert. Consumers should check back frequently because additional products may be added.
The following product is subject to the public health alert [view labels]:
12-oz. clear plastic tray packages labeled “MARKETSIDE LINGUINE WITH BEEF MEATBALLS & MARINARA SAUCE” with “best if used by” dates SEP 22, 2025; SEP 24, 2025; SEP 25, 2025; SEP 29, 2025; SEP 30, 2025; and OCT 01, 2025.
The product bears establishment number “EST. 50784” or "EST. 47718" inside the USDA mark of inspection. This item was shipped to Walmart locations nationwide.
The producing company collected samples of the FDA-regulated, pre-cooked pasta used as an ingredient in these products as part of the ongoing investigation related to the Listeria outbreak linked to chicken fettuccine alfredo meals. The test confirmed that the linguine pasta was positive for Lm and further testing is ongoing to determine if the Lm is genetically related to the specific outbreak strain. FSIS previously issued a recall notice linked to the Listeria outbreak in June and continues to coordinate with FDA, the Centers for Disease Control and Prevention, and state public health partners.
Consumption of food contaminated with Lm can cause listeriosis, a serious infection that primarily affects older adults, persons with weakened immune systems, and pregnant women and their newborns. Less commonly, persons outside these risk groups are affected.
Listeriosis can cause fever, muscle aches, headache, stiff neck, confusion, loss of balance and convulsions sometimes preceded by diarrhea or other gastrointestinal symptoms. An invasive infection spreads beyond the gastrointestinal tract. In pregnant women, the infection can cause miscarriages, stillbirths, premature delivery or life-threatening infection of the newborn. In addition, serious and sometimes fatal infections can occur in older adults and persons with weakened immune systems. Listeriosis is treated with antibiotics. Persons in the higher-risk categories who experience flu-like symptoms within two months after eating contaminated food should seek medical care and tell the health care provider about eating the contaminated food.
FSIS is concerned that some product may be in consumers’ refrigerators or freezers. Consumers who have purchased these products are urged not to consume them. These products should be thrown away or returned to the place of purchase.
Consumers with questions regarding the public health alert can contact Nate’s Fine Foods at 916-677-7303. Operating hours are between 9:00 a.m. and 4:00 p.m. PST Monday through Friday.
Consumers with food safety questions can call the toll-free USDA Meat and Poultry Hotline at 888-MPHotline (888-674-6854) or send a question via email to MPHotline@usda.gov. For consumers that need to report a problem with a meat, poultry, or egg product, the online Electronic Consumer Complaint Monitoring System can be accessed 24 hours a day at https://foodcomplaint.fsis.usda.gov/eCCF/.
Kroger bagged frozen shrimp and Kroger frozen shrimp products
Necessary Information:
See original announcement for examples of labels
Company Announcement:
Lawrence Wholesale LLC of Vernon, CA is recalling a limited quantity of Kroger bagged frozen shrimp and Kroger frozen shrimp products because they may have been prepared, packed, or held under insanitary conditions whereby they may have become contaminated with Cesium-137 (Cs-137).
Cs-137 is a man-made radioisotope of cesium. Traces of Cs-137 are widespread and can be present in the environment at background levels, and at higher levels in water or foods grown, raised, or produced in areas with environmental contamination. The primary health effect of concern following longer term, repeated low dose exposure (e.g., through consumption of contaminated food or water over time) is an elevated risk of cancer, resulting from damage to DNA within living cells of the body.
Bagged frozen shrimp and shrimp cocktail products were sold by Kroger in the following states: AK, AL, AR, AZ, CA, CO, GA, ID, IL, IN, KS, KY, LA, MI, MO, MS, MT, NE, NM, NV, OH, OR, SC, TN, TX, UT, VA, WA, WI, WV and WY. The product has the following descriptions and codes:
Item Description
UPC
Lot Number
Best By Date
Shrimp Bowl
Cooked Shrimp with
Cocktail Sauce (7oz)
011110622952
11325-H3A1
11425-H3A1
11525-H3A1
11625-H3A1
04/22/2027
04/23/2027
04/24/2027
04/25/2027
Shrimp Cocktail with
Cocktail Sauce
(17oz)
011110624840
13725-H3A1
13825-H3A1
05/16/2027
05/17/2027
Kroger Cooked
Jumbo Tail-On,
Peeled & Deveined
16/25 Shrimp
011110649812
11925-H3A1
12025-H3A1
04/28/2027
04/29/2027
Kroger Cooked
Medium Tail-On,
Peeled & Deveined
51/60 Shrimp
011110967015
12225-H3A1
12325-H3A1
05/01/2027
05/02/2027
The FDA is actively investigating reports of Cs-137 contamination in shipping containers and frozen shrimp products manufactured in Indonesia by PT. Bahari Makmur Sejati (doing business as BMS Foods). No illnesses have been reported to date. As noted in the FDA statement issued on 8/19/25: “At this time, no product that has tested positive or alerted for Cesium-137 (Cs-137) has entered the U.S. commerce.”
FDA is working with distributors and retailers that received product from PT. Bahari Makmur Sejati after the date of first detection of Cs-137 by Customs & Border Protection (CBP), but from shipments that did not alert for Cs-137, to recommend that firms conduct a recall. In conjunction with other information, FDA determined that product from PT. Bahari Makmur Sejati violates the Federal Food, Drug, & Cosmetic Act in that it appears to have been prepared, packed, or held under insanitary conditions whereby it may have become contaminated with Cs-137 and may pose a safety concern.
Consumers who have purchased the recalled bagged frozen shrimp or frozen shrimp products should not consume the product and should dispose of the product or return it to the place of purchase for a full refund. Consumers with questions may contact the company at (323) 235-7535, Monday to Friday from 9:00 am to 4:00 pm PT.
This recall is being made with the knowledge of the U.S. Food and Drug Administration.
Readt to eat tirkey gobbler wrap
Necessary Information:
See original announcement for examples of labels
Company Announcement:
WASHINGTON, September 19, 2025 – The U.S. Department of Agriculture’s Food Safety and Inspection Service (FSIS) is issuing a public health alert for a ready-to-eat (RTE) turkey wrap product due to concerns that the product may be contaminated with Listeria monocytogenes. A recall was not requested because the affected product is no longer available for purchase.
The RTE turkey wrap product was produced on September 10, 2025. The following product is subject to the public health alert [view sample labels]:
10-oz. clear plastic clamshell packages containing “TRADER JOE’S TURKEY GOBBLER WRAP” with “BEST BY SEP 16, 2025.”
The product bears establishment number “P-1644” inside the USDA mark of inspection. This item was shipped to Trader Joe’s retail locations nationwide.
The problem was discovered when the establishment notified FSIS that food contact surface samples associated with this product tested positive for Listeria monocytogenes.
There have been no confirmed reports of adverse reactions due to consumption of this product. Anyone concerned about an illness should contact a health care provider.
Consumption of food contaminated with L. monocytogenes can cause listeriosis, a serious infection that primarily affects older adults, persons with weakened immune systems, and pregnant women and their newborns. Less commonly, persons outside these risk groups are affected.
Listeriosis can cause fever, muscle aches, headache, stiff neck, confusion, loss of balance and convulsions sometimes preceded by diarrhea or other gastrointestinal symptoms. An invasive infection spreads beyond the gastrointestinal tract. In pregnant women, the infection can cause miscarriages, stillbirths, premature delivery or life-threatening infection of the newborn. In addition, serious and sometimes fatal infections can occur in older adults and persons with weakened immune systems. Listeriosis is treated with antibiotics. Persons in the higher-risk categories who experience flu-like symptoms within two months after eating contaminated food should seek medical care and tell the health care provider about eating the contaminated food.
FSIS is concerned that some product may be in consumers’ refrigerators. Consumers who have purchased this product are urged not to consume it. The product should be thrown away or returned to the place of purchase.
Media and consumers with questions regarding the public health alert can contact Trader Joe’s Customer Relations at 626-599-3817, on Monday through Friday from 6:00 am to 6:00 pm Pacific Time.
Consumers with food safety questions can call the toll-free USDA Meat and Poultry Hotline at 888-MPHotline (888-674-6854) or send a question via email to MPHotline@usda.gov. For consumers that need to report a problem with a meat, poultry, or egg product, the online Electronic Consumer Complaint Monitoring System can be accessed 24 hours a day at https://foodcomplaint.fsis.usda.gov/eCCF/
Kroger Mercado Cooked Medium Peeled Tail-Off Shrimp.
Necessary Information:
See original announcement for examples of labels
Company Announcement:
September 19, 2025, AquaStar (USA) Corp of Seattle, WA is recalling:
approximately 49,920 bags (net wt. 2lbs) of Kroger Raw Colossal EZ Peel Shrimp.
approximately 18,000 bags (net wt. 2lbs) of Kroger Mercado Cooked Medium Peeled Tail-Off Shrimp.
approximately 17,264 bags (net wt. 1.25lbs) of AquaStar Raw Peeled Tail-on Shrimp Skewers because they may have been prepared, packed, or held under insanitary conditions whereby they may have become contaminated with cesium-137 (Cs-137).
Cs-137 is a man-made radioisotope of cesium. Traces of Cs-137 are widespread and can be present in the environment at background levels, and at higher levels in water or foods grown, raised, or produced in areas with environmental contamination. The primary health effect of concern following longer term, repeated low dose exposure (e.g., through consumption of contaminated food or water over time) is an elevated risk of cancer, resulting from damage to DNA within living cells of the body.
The affected shrimp was sold at Baker’s, City Market, Dillons, Food 4 Less, Foodsco, Fred Meyer, Fry’s, Gerbes, Jay C, King Soopers, Kroger, Mariano’s, Metro Market, Pay Less Supermarkets, Pick ‘n Save, Ralphs, Smith’s and QFC in AK, AL, AR, AZ, CA, CO, GA, ID, IL, IN, KS, KY, LA, MI, MO, MS, MT, NE, NM, NV, OH, OR, SC, TN, TX, UT, VA, WA, WI, WV and WY between June 12, 2025 and September 17, 2025.
The recalled Kroger Raw Colossal EZ Peel Shrimp net wt. 2lbs., is packaged in transparent printed bag with a blue band on the top with yellow and red details, and has the following codes:
UPC 20011110643906, lot code 10662 5085 10, Best If Used By: 03 26 27
UPC 20011110643906, lot code 10662 5097 11, Best If Used By: 04 07 27
UPC 20011110643906, lot code 10662 5106 11, Best If Used By: 04 16 27
UPC 20011110643906, lot code 10662 5107 10, Best If Used By: 04 17 27
UPC 20011110643906, lot code 10662 5111 11, Best If Used By: 04 21 27
UPC 20011110643906, lot code 10662 5112 10, Best If Used By: 04 22 27
UPC 20011110643906, lot code 10662 5113 10, Best If Used By: 04 23 27
UPC 20011110643906, lot code 10662 5113 11, Best If Used By: 04 23 27
UPC 20011110643906, lot code 10662 5114 10, Best If Used By: 04 24 27
UPC 20011110643906, lot code 10662 5114 11, Best If Used By: 04 24 27
The recalled Kroger Mercado Cooked Medium Peeled Tail-Off Shrimp, net wt. 2lbs., is packaged in clear plastic bag and has a white label with green stripes on top of each bag and has the following codes:
UPC 011110626196, lot code 10662 5112 11, Best Before: 10 22 2027
UPC 011110626196, lot code 10662 5113 10, Best Before: 10 23 2027
The recalled AquaStar Raw Peeled Tail-on Shrimp Skewers; net wt. 1.25 lbs., is packaged in a printed bag with a black top and blue bottom and printed pictures of the skewers inside, and has the following codes:
UPC 731149390010, lot code 10662 5127 10, Best If Used By: 11 07 2027
UPC 731149390010, lot code 10662 5128 11, Best If Used By: 11 08 2027
UPC 731149390010, lot code 10662 5133 11, Best If Used By: 11 13 2027
UPC 731149390010, lot code 10662 5135 10, Best If Used By: 11 15 2027
The FDA is actively investigating reports of Cesium-137 (Cs-137) contamination in shipping containers and frozen shrimp products processed by PT. Bahari Makmur Sejati (doing business as BMS Foods) of Indonesia. No illnesses have been reported to date. As noted in the FDA statement issued on 8/19/25: “At this time, no product that has tested positive or alerted for Cesium-137 (Cs-137) has entered the U.S. commerce.”
“FDA is working with distributors and retailers that received product from PT. Bahari Makmur Sejati after the date of first detection of Cs-137 by Customs & Border Protection (CBP), but from shipments that did not alert for Cs-137, to recommend that firms conduct a recall. In conjunction with other information, FDA determined that product from PT. Bahari Makmur Sejati violates the Federal Food, Drug, & Cosmetic Act in that it appears to have been prepared, packed, or held under insanitary conditions whereby it may have become contaminated with Cs-137 and may pose a safety concern.”
Consumers who have purchased affected shrimps should not consume the product and should dispose of or return it to the place of purchase for a full refund.
Consumers with questions may contact the company at 1-800-331-3440, Monday-Friday, 8am-5 pm PST.
This recall is being made with the knowledge of the U.S. Food and Drug Administration
Kirkland Signature brand Ahi Tuna Wasabi Poke
Necessary Information:
See original announcement for examples of labels
Company Announcement:
September 20, 2025, Western United Fish Company dba Annasea Foods Group of Kent, WA is recalling 3,314.7 lbs. of Kirkland Signature brand Ahi Tuna Wasabi Poke, Costco Item Number of 17193, with the Sell By Date of 9/22/2025, because the green onions used in the product have the potential to be contaminated with Listeria monocytogenes, an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Although healthy individuals may suffer only short-term symptoms such as high fever, severe headache, stiffness, nausea, abdominal pain and diarrhea, Listeria infection can lead to serious pregnancy complications among pregnant women.
The affected Ahi Tuna Wasabi Poke product is packaged in clear plastic clamshell containers and has the Kirkland Signature brand label with the Pack Date of 9/18/2025 and Sell By Date of 9/22/2025.
Product was sold at the deli section from Costco Warehouse stores in the following states on 9/18/2025: Alabama, Alaska, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Indiana, Louisiana, Maine, Maryland, Massachusetts, Minnesota, Mississippi, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, Ohio, Oregon, Pennsylvania, South Carolina, Tennessee, Texas, Utah, Virginia, Washington, and Wisconsin.
No illnesses have been reported to date.
This recall was initiated after being notified by our green onion supplier of a Listeria monocytogenes positive test result in the green onions which were used only in Ahi Tuna Wasabi Poke on 9/17/2025. We are continuing to work with our green onion supplier to determine the root cause.
Consumers should not consume this affected product and dispose of it immediately and visit your local Costco for a full refund.
Please call Western United Fresh Co. DBA Annasea Foods Group at (425) 558-7809, 7:00am - 3:30pm Pacific Time, Monday – Friday, or email info@annasea.com if you have any issues or concerns.
This recall is being made with the knowledge of the U.S. Food and Drug Administration.
Sprout Organics® Sweet Potato Apple and Spinach
Necessary Information:
See original announcement for examples of labels
Company Announcement:
Sprout Organics has initiated a voluntary recall of one lot of Sprout Organics® Sweet Potato Apple and Spinach because it may contain elevated levels of lead.
Exposure to lead, even at low levels, may increase blood lead levels. Additional signs and symptoms of lead exposure are more likely with acute exposure to higher levels of lead or chronic exposure to lead. The effects of lead depend upon the amount and duration of exposure and age/body weight. If a child is exposed to enough lead for a protracted period of time, this can affect learning and development or cause other long-term health problems.
The product, a 3.5-ounce pouch, was sold in Walgreens and some independent stores in the US South region with most sales occurring between September and December 2024. The product was not sold in any other large grocery chain besides Walgreens.
If consumers have product matching the following description in their possession, they should return it to their local store for a full refund. The lot code and expiration date are printed on the bottom strip on the back of the pouch (see photos attached).
Product Name
Purchased From
Lot Code
Expiration Date
Sprout Organics® Sweet
Potato Apple and Spinach
3.5 oz pouch
Walgreens and independent
stores in US South region
4212
10/29/2025
No illnesses have been reported to date and no other Sprout Organics products are impacted by this voluntary recall. The voluntary recall is being initiated after routine sampling.
To learn about Sprout Organics comprehensive testing program of ingredients and products before distribution, please go to https://sproutorganics.com/pages/faqExternal Link Disclaimer.
Consumers with questions should contact the company at 510-833-6089 Monday through Friday 9 am to 5 pm Pacific Time or by email at Info@sproutorganics.com.
RECALL Good & Gather Southwest-Style Burrito Bowl Blend
Necessary Information:
See original announcement for examples of labels
Company Announcement:
One Frozen, LLC of Rochester, NY, is voluntarily recalling certain lots of Good & Gather™ Southwest-Style Burrito Bowl Blend (frozen, 12oz bags) because the product may contain shrimp (crustacean shellfish) that was not declared on the ingredient label of the product. People who have an allergy or severe sensitivity to shellfish run the risk of a serious or life-threatening allergic reaction if they consume this product.
Good & Gather™ Southwest-Style Burrito Bowl Blend were distributed through Target retail stores nationwide, in all 50 states. The distribution from One Frozen, LLC to Target began on 4/17/2025.
Product Identification:
Product Name: Good & Gather™ Burrito Bowl Blend, Southwest-Style (Frozen)
Package Size: 12 oz (340g) bags
UPC: 085239931356 – Lot codes can be found under the Nutrition Label on the back of the packaging
Lot Codes: L5055-1, L5055-2, L5055-3, L5055-4, L5055-5, and L5055-6
Best if Used by Date: 08/24/2026
Quantity Distributed: 57,240 consumer units
The recall is being initiated after three consumer complaints were received indicating the possible presence of shrimp in this product. No illness have been reported as of September 10, 2025.
This recall is being conducted in cooperation with the U.S. Food and Drug Administration (FDA).
What Consumers Should Do:
Check your freezer for the affected product.
If you purchased the Good & Gather™ Southwest-Style Burrito Bowl Blend, 12 oz bag with lot codes L5055-1, L5055-2, L5055-3, L5055-4, L5055-5, and L5055-6, do not consume this product and contact Target Guest Relations at (800) 440-0680 for a full refund.
Kroger Mercado Cooked Medium Peeled Tail-Off Shrimp recall
****RECALL****
MISSOURI DEPARTMENT OF HEALTH AND SENIOR SERVICES RECALL ALERT
Necessary Information:
• Web Link- https://www.fda.gov/.../aquastar-usa-corp-recalls-kroger...
• See original announcement for examples of labels
Company Announcement
August 27, 2025, AquaStar (USA) Corp of Seattle, WA is recalling approximately 18,000 bags (net wt. 2lbs) of Kroger Mercado Cooked Medium Peeled Tail-Off Shrimp because they may have been prepared, packed, or held under insanitary conditions whereby they may have become contaminated with cesium-137 (Cs-137).
Cs-137 is a man-made radioisotope of cesium. Traces of Cs-137 are widespread and can be present in the environment at background levels, and at higher levels in water or foods grown, raised, or produced in areas with environmental contamination. The primary health effect of concern following longer term, repeated low dose exposure (e.g., through consumption of contaminated food or water over time) is an elevated risk of cancer, resulting from damage to DNA within living cells of the body.
The affected shrimp was sold at Baker’s, Gerbes, Jay C, Kroger, Mariano’s, Metro Market, Pay Less Supermarkets, and Pick ‘n Save in AL, AR, GA, IL, IN, KS, KY, MI, MO, MS, NE, OH, SC, TN, VA, WI, WV between July 24, 2025 and August 11, 2025.
The recalled Kroger Mercado Cooked Medium Peeled Tail-Off Shrimp, net wt. 2lbs., is packaged in clear plastic bag and has a white label with green stripes on top of each bag and has the following codes:
• UPC 011110626196, Lot code 10662 5139, Best Before 11/19/2027
• UPC 011110626196, Lot code 10662 5140, Best Before 11/20/2027
The FDA is actively investigating reports of Cesium-137 (Cs-137) contamination in shipping containers and frozen shrimp products processed by PT. Bahari Makmur Sejati (doing business as BMS Foods) of Indonesia. No illnesses have been reported to date. As noted in the FDA statement issued on 8/19/25: “At this time, no product that has tested positive or alerted for Cesium-137 (Cs-137) has entered the U.S. commerce.”
“FDA is working with distributors and retailers that received product from PT. Bahari Makmur Sejati after the date of first detection of Cs-137 by Customs & Border Protection (CBP), but from shipments that did not alert for Cs-137, to recommend that firms conduct a recall. In conjunction with other information, FDA determined that product from PT. Bahari Makmur Sejati violates the Federal Food, Drug, & Cosmetic Act in that it appears to have been prepared, packed, or held under insanitary conditions whereby it may have become contaminated with Cs-137 and may pose a safety concern.”
Consumers who have purchased affected shrimps should not consume the product and should dispose of or return it to the place of purchase for a full refund.
Consumers with questions may contact the company at 1-800-331-3440, Monday-Friday, 8am-5 pm PST.
This recall is being made with the knowledge of the U.S. Food and Drug Administration.
Taylor Farms Honey Balsamic Salad Kit recall
****RECALL****
Necessary Information:
• Web Link- https://www.fda.gov/.../company-voluntarily-recalls-honey...
• See original announcement for examples of labels
Company Announcement:
Salinas, Calif., August 26, 2025 – In response to a recall initiated by Latitude 36 Foods, LLC., Taylor Fresh Foods is voluntarily recalling the Taylor Farms Honey Balsamic Salad Kit 6/8.3oz. because it may contain undeclared sesame and soy allergens. People who have an allergy or severe sensitivity to sesame and soy run the risk of serious or life-threatening allergic reaction if they consume these products.
Master packs — individual packets of dressing and toppings supplied by Latitude 36 Foods and included in Taylor Farms salad kits — incorrectly included Asian Sesame Ginger dressing rather than the intended Honey Balsamic Vinaigrette dressing, leading to the possibility of undeclared sesame and soy allergens in some Taylor Farms Honey Balsamic Salad Kits.
The Taylor Farms Honey Balsamic Salad Kit 6/8.3oz in scope of this recall was distributed in AL, AZ, CA, CO, DE, FL, GA, IN, KS, KY, LA, MI, MO, MS, NJ, NY, OH, OR, PA, TN, TX, UT, VA, WA and WV and has code dates starting with “TFRS” and “Best If Used By” date up to and including September 4, 2025. The product code can be found in the upper right-hand corner of the packaging.
Consumers who have the recalled salad kit should discard it immediately and not consume it. Refunds are available at the location of purchase. There have been no illnesses reported to Taylor Farms in connection with the recalled product. This recall does not apply to any other Taylor Farms products or brands. Consumers with any questions may contact our customer care team at 855-455-0098 Monday through Friday from 8am-5pm PT.
• Distribution information:
This product was distributed to the following Walmart stores
Address
City
State
Zip Code
1310 PREACHER ROE BLVD
WEST PLAINS
MO
65775-2938
333 S WESTWOOD BLVD
POPLAR BLUFF
MO
63901-5519
2050 W 76 COUNTRY BLVD
BRANSON
MO
65616-2100
1500 1ST ST
KENNETT
MO
63857-2522
1401 OLD EXETER ROAD
CASSVILLE
MO
65625-9415
18401 STATE HWY 13
BRANSON WEST
MO
65737-0000
1101 BRANSON HILLS PKWY
BRANSON
MO
65616-9942
100 COMMERCIAL LN
PINEVILLE
MO
64856-7069
3001 OAK GROVE ROAD
POPLAR BLUFF
MO
63901-0000
blue bell icecream recall
****RECALL****
Necessary Information:
• Web Link- https://www.fda.gov/.../blue-bell-ice-cream-issues...
• See original announcement for examples of labels
Company Announcement:
BRENHAM, Texas, August 22, 2025 – Blue Bell Ice Cream is voluntarily recalling a limited quantity of Moo-llennium Crunch Ice Cream half gallon packaged in a Chocolate Chip Cookie Dough carton produced in its Brenham, Texas, plant because of undeclared almond, walnut, and pecan. The recalled product was mistakenly packaged in Chocolate Chip Cookie Dough ice cream cartons with a Moo-llennium Crunch lid. People who have an allergy or severe sensitivity to almonds, walnuts, and pecans run the risk of a serious or life-threatening allergic reaction if they consume these products.
A Blue Bell employee discovered the incorrect packaging on two half gallons while restocking a retailer. No illnesses or adverse reactions have been reported to date. No other incorrect packaging has been discovered or reported to date.
The half gallons can be identified as Moo-llennium Crunch Ice Cream packaged in a Chocolate Chip Cookie Dough half gallon carton with a Moo-llennium Crunch lid and with the following code located on the top of the half gallon lid: 061027524. An image of the affected product is included below.
The affected ice cream half gallons were distributed through retail outlets in Alabama, Arkansas, Florida Panhandle, Northwest Georgia, Southern Indiana, Southern Illinois, Kansas, Kentucky, Louisiana, Mississippi, Missouri, New Mexico, Oklahoma, Tennessee, Texas, and Southwest Virginia.
Consumers who have purchased these items can return them to the place of purchase for a full refund.
For more information, consumers may call 979-836-7977, Monday – Friday 8 a.m. – 5 p.m. CST or email us at consumerrelations@bluebell.com.
This recall is being made with the knowledge of the U.S. Food and Drug Administration.
RECALL Raw Frozen Shrimp PT. Bahari Makmur Sejati (doing business as BMS Foods)
Necessary Information:
See original announcement for examples of labels
Company Announcement:
Product and stores affected
Certain raw frozen shrimp products processed by PT. Bahari Makmur Sejati (doing business as BMS Foods), a company located in Indonesia, and sold at Walmart.
These products include the following product names, lot codes, and best by dates:
Great Value brand frozen raw shrimp, lot code: 8005540-1, Best by Date: 3/15/2027
Great Value brand frozen raw shrimp, lot code: 8005538-1, Best by Date: 3/15/2027
Great Value brand frozen raw shrimp, lot code: 8005539-1, Best by Date: 3/15/2027
Additional product information is in a table below.
At this time, no product that has tested positive or alerted for Cesium-137 (Cs-137) has entered the U.S. commerce. FDA is working with distributors and retailers that received product from PT. Bahari Makmur Sejati after the date of first detection of Cs-137 by Customs & Border Protection (CBP), but from shipments that did not alert for Cs-137, to recommend that firms conduct a recall. In conjunction with other information, FDA determined that product from PT. Bahari Makmur Sejati violates the Federal Food, Drug, & Cosmetic Act in that it appears to have been prepared, packed, or held under insanitary conditions whereby it may have become contaminated with Cs-137 and may pose a safety concern.
FDA has also added PT. Bahari Makmur Sejati to a new import alert for chemical contamination to stop products from this firm from coming into the U.S. until the firm has resolved the conditions that gave rise to the appearance of the violation.
FDA’s investigation is ongoing. This advisory will be updated as more information becomes available.
Health impacts of cesium exposure
FDA detected Cs-137 in a single shipment of imported frozen shrimp from PT. Bahari Makmur Sejati that did not enter U.S. commerce. The level of Cs-137 detected in the detained shipment was approximately 68 Bq/kg, which is below FDA’s Derived Intervention Level for Cs-137 of 1200 Bq/kg. At this level, the product would not pose an acute hazard to consumers. Avoiding products like the shipment FDA tested with similar levels of Cs-137 is a measure intended to reduce exposure to low-level radiation that could have health impacts with continued exposure over a long period of time.
The primary health effect of concern following longer term, repeated low dose exposure (e.g., through consumption of contaminated food or water over time) is an elevated risk of cancer, resulting from damage to DNA within living cells of the body. Additional information about Cs-137 and your health is available through the Centers for Disease Control and Prevention’s Agency for Toxic Substances and Disease Registry resources.
Recommendation
If you recently purchased one of the impacted lots of Great Value raw frozen shrimp from Walmart, throw it away. Do not eat or serve this product.
Distributors and retailers should dispose of this product and should not sell or serve this product.
If you suspect you have been exposed to elevated levels of cesium, talk to your healthcare provider.
Current Update
August 19, 2025
FDA is actively investigating reports of Cesium-137 (Cs-137) contamination in shipping containers and frozen shrimp products processed by PT. Bahari Makmur Sejati (doing business as BMS Foods) of Indonesia. The U.S. Customs & Border Protection (CBP) alerted FDA to the detection of Cs-137 in shipping containers at four U.S. ports (Los Angeles, Houston, Savannah, and Miami). FDA collected multiple samples for radionuclide analysis, with results confirming the presence of Cs-137 in one sample of breaded shrimp. All containers and product testing positive or alerting for Cs-137 have been denied entry into the country. The agency continues to coordinate with CBP to prevent any contaminated products from reaching consumers and is working with Indonesian seafood regulatory authorities to investigate the root cause of the contamination.
Although testing to date has not confirmed the presence of contamination in any product in commerce, the product appears to have been prepared, packed, or held under insanitary conditions whereby it may have become contaminated with Cs-137 and may pose a safety concern. To date, FDA has learned that Walmart has received implicated raw frozen shrimp, imported after the date of first detection of Cs-137 by CBP, but from shipments that did not alert for Cs-137. FDA has recommended Walmart recall this product.
Consumers should not eat or serve certain lots of Great Value raw frozen shrimp from Walmart:
Great Value brand frozen raw shrimp, lot code: 8005540-1, Best by Date: 3/15/2027
Great Value brand frozen raw shrimp, lot code: 8005538-1, Best by Date: 3/15/2027
Great Value brand frozen raw shrimp, lot code: 8005539-1, Best by Date: 3/15/2027.
If you have recently purchased raw frozen shrimp from Walmart that matches this description, throw it away. Do not eat or serve this product.
Cs-137 is a radioisotope of cesium that is man-made through nuclear reactions and because it is widespread worldwide, trace amounts of Cs-137 can be found in the environment, including soil, food, and air. FDA food monitoring focuses on radioisotopes (radionuclides) that are not normally present and are generally the result of human activities. Any unexpected finding of Cs-137 in a food product is evaluated to determine if follow up action is warranted on a case-by-case basis. After being alerted to the contamination of shipping containers detected by CBP, FDA initiated sampling of products which included five different shrimp products from PT. Bahari Makmur Sejati, one of which was a sample of frozen breaded shrimp. FDA's laboratory confirmation of Cs-137 in the breaded shrimp had detectable levels of Cs-137 present at 68.48 Bq/kg +/- 8.25 Bq/kg. There was no detectable Cs-137 in the other products tested; however, this does not rule out contamination.
FDA has not detected Cs-137 in any product above the current derived intervention levels for Cs-137 (1200 Bq/kg); however, FDA has concluded that the level detected in the breaded shrimp sample could represent a potential health concern for those exposed to this level of Cs-137 from consumption of the shrimp over an extended period of time combined with radiation that exists in the environment and from other sources such as medical procedures. Avoiding products like the shipment FDA tested with similar levels of Cs-137 is a measure intended to reduce exposure to low-level radiation that could have health impacts with continued exposure over a long period of time. FDA has taken swift action to prevent potentially contaminated product from being introduced into U.S. commerce in response to reports of contaminated shipping containers from CBP. On August 14, 2025, FDA posted a new import alert (IA 99-51) for chemical contamination under section 402(a)(4) of the Federal Food, Drug, and Cosmetic Act. PT. Bahari Makmur Sejati has been added to the red list of this alert due to the presence of Cs-137. The import alert ensures that no implicated shrimp products will enter U.S. commerce until the company resolves the conditions that gave rise to the appearance of the violation.
FDA will continue working with industry to trace all implicated products processed by PT. Bahari Makmur Sejati through the supply chain to gather as much information about them as possible and take action as appropriate. Product information will be added to this advisory as it becomes available.
Product Descriptions
Brand
Product Name
Product Type
Best By
Item Code
Lot Code
Distributor
Great Value
Frozen Raw White Vannamei Shrimp
Frozen Raw Ez Peel Tail-On Farm-Raised White Vannamei Shrimp, 2lb bag
3/15/2027
7383108
8005540-1
Walmart
Great Value
Frozen Raw White Vannamei Shrimp
Frozen Raw Ez Peel Tail-On Farm-Raised White Vannamei Shrimp, 2lb bag
3/15/2027
7383108
8005538-1
Walmart
Great Value
Frozen Raw White Vannamei Shrimp
Frozen Raw Ez Peel Tail-On Farm-Raised White Vannamei Shrimp, 2lb bag
3/15/2027
7383108
8005539-1
Walmart
Useful Links
Clover Valley Instant Coffee Recall
• Web Link- https://www.fda.gov/.../dollar-general-announces...
• See original announcement for examples of labels
Company Announcement:
Goodlettsville, Tennessee – August 11, 2025 –Dollar General Corporation is recalling three (3) lots of its eight ( ounce Clover Valley® Instant Coffee due to the potential presence of glass.
8-Ounce Clover Valley® Instant Coffee
Package UPC: 876941004069
Lot: L-5163 / Best By 12/13/2026
Lot: L-5164 / Best by 12/13/2026
Lot: L-5165 / Best by 12/14/2026
Customers can find the lot and best by date information around the neck of the unit.
Clover Valley® Instant Coffee was sold and distributed between July 9-21, 2025 exclusively in Dollar General retail stores located in the following states: AL, AR, AZ, CA, CO, CT, DE, FL, GA, IA,ID, IL, IN, KS, KY, LA, MA, MD, ME, MI, MN, MO, MS, MT, NC, ND, NE, NH, NJ, NM, NV, NY, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VA, VT, WA, WI ,WV, and WY.
The recall is being initiated after a customer notified Dollar General employees about the potential issue. Ingesting glass fragments may cause injury to the consumer, and these injuries may include damage to teeth, laceration of the mouth and throat, or perforation of the intestine. No illnesses or injuries have been reported to date.
Customers who purchased this product are encouraged to discard it and contact Dollar General either via email at customercare@dollargeneral.com or by phone at 1-888-309-9030 from 6 a.m. to 1 a.m. CST seven days a week to request a full refund of the purchase price (including any tax).
Dollar General is actively investigating the source of the glass contamination and apologizes for any inconvenience caused by this product issue. The recall is being conducted with the knowledge of the U.S. Food & Drug Administration (FDA).
For additional information, please contact the Media Relations Department at dgpr@dg.com.
About Dollar General Corporation Dollar General Corporation
(NYSE: DG) is proud to serve as America’s neighborhood general store. Founded in 1939, Dollar General lives its mission of Serving Others every day by providing access to affordable products and services for its customers, career opportunities for its employees, and literacy and education support for its hometown communities. As of May 2, 2025, the Company’s 20,582 Dollar General, DG Market, DGX and pOpshelf stores across the United States and Mi Súper Dollar General stores in Mexico provide everyday essentials including food, health and wellness products, cleaning and laundry supplies, self-care and beauty items, and seasonal décor from our high-quality private brands alongside many of the world’s most trusted brands such as Coca Cola, PepsiCo/Frito-Lay, General Mills, Hershey, J.M. Smucker, Kraft, Mars, Nestlé, Procter & Gamble and Unilever.
DermaRite Industries recall
Necessary Information:
· Web Link- https://www.fda.gov/.../dermarite-industries-issues...
· See original announcement for examples
Company Announcement
DermaRite Industries, LLC is voluntarily recalling individual lots of products in the table below due to microbial contamination identified as Burkholderia cepecia.
Risk Statement: Burkholderia Cepacia Complex in these products may result in serious and life-threatening infections. The contaminated products may be used by immunosuppressed individuals or by people attending to immunosuppressed individuals. In healthy individuals with minor skin lesions the use of the product will more likely result in local infections, whereas in immunocompromised individuals the infection is more likely to spread into blood stream leading to life-threatening sepsis. To date, DermaRite has not received any reports of adverse events related to this recall.
DermaKleen is an OTC Healthcare antiseptic lotion soap with Vitamin E indicated for handwashing to decrease bacteria on the skin.
DermaSarra is an OTC External analgesic indicated for temporary relief of itching associated with minor skin irritations due to: dry skin, insect bites, detergents, sunburn.
KleenFoam is an OTC Antimicrobial foam soap with Aloe Vera indicated for handwashing to decrease bacteria on the skin after changing diapers, after assisting ill people, or before contact with a person under medical care or treatment.
PeriGiene is an OTC Antiseptic cleanser indicated for use in the perineal area.
The recalled products were distributed nationwide in the United States and in Puerto Rico.
Recalled Product Information: (see Table image below)
DermaRite has notified its distributors and customers by e-mail to immediately examine available inventory and destroy all affected products in accordance with each facility’s process.
Consumers with questions regarding this recall can call Mary Goldberg at 973-569-9000 x104 Monday through Friday, 9:00 am – 5:00 pm EST or email voluntary.action@dermarite.com.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail, or by fax.
• Complete and submit the report online: www.fda.gov/medwatch/report.htm
• Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
Consumers:
Mary Goldberg
973-569-9000 x104
voluntary.action@dermarite.com